Phase Change Materials
Phase change materials (PCM) have gained a lot of attention in recent years for thermal management of systems as well as energy storage. In phase change, heat is transferred through absorption and rejection of heat by the phase changing medium. Phase change may take place from solid to liquid or from liquid to gas. Over the years, both types of phase changes have found applications in electronic cooling and other applications.
In this article, we will discuss the theory behind phase change, the types of applications currently using phase change, and the phase change materials available in the market today.
Basics of Phase Change Materials
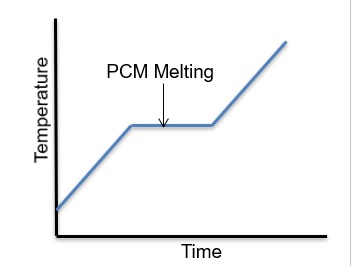
When materials change phase from solid to liquid or from liquid to gas, they absorb heat. Likewise, when PCMs change phase from gas to liquid or from liquid to solid, they release heat to the surroundings. The heat absorbed or released per unit mass of the material is called Latent Heat. Different materials have different values of latent heat. Some materials have high latent heat, some low. The latent heat has the same value during either way of the phase change direction.
The basic equation governing phase change is:
VρL = Pt
Where,
V = Volume of PCM
ρ = Density of PCM
L = Latent heat of fusion of PCM (per Mass)
P = Power absorbed, W
t = Time of power absorption, seconds
The above equation basically states that the heat storage capability of a phase change material is proportional to the mass of the material and the latent heat of the material. In other words, to store a large amount of heat, there are two ways of doing it. We must have either a large mass of PCM and/or a large latent heat. Because space is always an issue in most thermal applications, high latent heat is, therefore, a very desirable property of a PCM material.
In addition to the latent heat, other desirable properties of PCM, according to our thermal management consultants, are the right melting temperature, high thermal conductivity, and chemical stability.
The melting temperature is important because it has to be relevant to the device we are trying to temperature-control. For example, an IC chip that needs to be maintained at 90C cannot use a PCM material that melts at 150C. The PCM material must change phase around 90C.
High Thermal conductivity is another desirable property of a PCM material. The reason is that the PCM must be thermally conducting enough so that the heat propagates through the PCM itself to melt it fully and quickly. Otherwise, the PCM will not melt fully near a given temperature – due to the large temperature gradient present inside the PCM. Some of the most common PCMs, such as paraffin wax, have low thermal conductivities (~0.2 W/mK). This is pretty low for many applications. Various strategies are, therefore, needed to enhance their thermal conductivities, including microencapsulation and macroencapsulation with more conducting materials, using more conducting structures (such as grids) inside PCM bodies, etc. In microencapsulation, the PCM is contained inside small spheres or capsules, which then can be embedded inside bulk materials, such as gypsum for construction use.
The PCM must also be non-corrosive so it does not affect the enclosure material containing the PCM or damage electronic parts in case of any leakage. Salt hydrates are less popular for this reason due to concerns with long-term reliability. PCM should also not be toxic to the user when exposed. Highly corrosive and/or toxic PCMs are, therefore, undesirable in most cases because of these issues. However, modern encapsulation technics are reducing these concerns as we speak, increasing their adoption in many industries.
Another very important factor with PCMs is the ability to melt congruently. To use the same PCM over a great many cycles lasting years, the PCM must melt congruently. Incongruent melting occurs when a solid substance does not melt uniformly but transforms itself into a different composition or form. This means the PCM cannot be used in the same form in subsequent cycles of melting and freezing. Such PCMs are undesirable in commercial applications.
Supercooling is another issue in phase change materials. In supercooling, the PCM in its liquid state can be cooled below its freezing point while remaining a liquid. For example, some salt hydrates can be cooled to 50 °C (122 °F) below their freezing point without crystallizing. For most applications, supercooling must be kept to a minimum by the addition of suitable nucleating agents to the PCM.
Finally, the cost of the PCM is a factor in most applications. In general, the PCM must be relatively inexpensive and widely available. For large systems, in particular, where huge volumes of PCM are used, such as in buildings, the cost of the PCM must be comparable to other materials they are likely to replace.
In many cases, weight is also a factor. In general, heavier PCMs are less desirable than lighter ones.
For more on PCM selection factors or help selecting phase change materials, please contact our thermal design consultants.
Available Phase Change Materials
Depending on the melting temperature, there are hundreds of phase change materials that can be used in one form or the other as recommended by thermal consultants. Basically, any material melts and freezes. The only question is whether a given PCM can be used for a certain application or not. The figure given below shows some of the phase change materials melting between -100 to 800+ C. Each circle/ellipse shows the range of melting temperature and latent heat associated with each PCM.
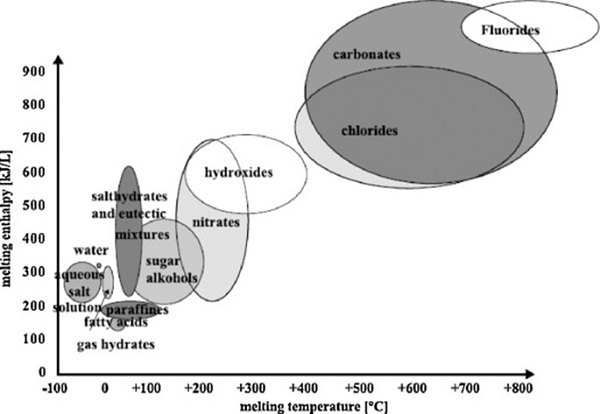
The melting temperature required depends on the application. For some applications, such as medical, the melting temperature required may be in the sub-zero degree C range. For building applications, the desirable melting temperatures may be room temperatures in the range 20-30C. For most electronic cooling applications, the useful melting temperature range can be 50-90C. For electricity generation applications, one may need materials that melt at much higher temperatures, like 250C using materials such as a mixture of Potassium Nitrade and Sodium Nitrade. There are many PCMs that melt and solidify at moderate temperatures, making them attractive for one application or the other. They include inorganic salt hydrates, paraffin waxes, fatty acids, and inorganic metal eutectics, (Fig. 3).
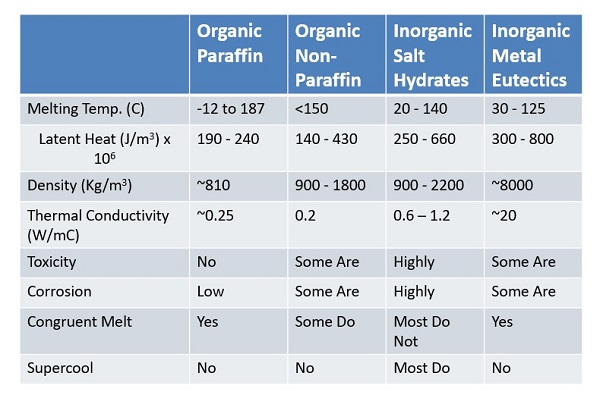
Of the above four categories, organic paraffin is the most well-known and most widely used. Paraffin waxes are chemically stable, readily available and inexpensive, and melt at temperatures of interest for many common applications. The main drawbacks of paraffin waxes are two: their latent heat is relatively low, and their thermal conductivity is low as well. Inorganic salt hydrates are superior to paraffin in these two areas, except that their chemical behavior (including toxicity and corrosion) is poor. Also, most do supercool and melt incongruently. Metal PCMs, another class of PCMs, have great properties overall, but weight and cost can be issues in many applications.
PCM for Electronic Cooling Applications
PCMs are best suited in applications where power inherently fluctuates with time, including in electronic cooling applications. They are not meant to handle steady power dissipations over long periods of time. The power fluctuation may occur during short time intervals, such as minutes, or during longer time intervals, such as days. When the temperature peaks, the PCM melts, storing the excess energy. When the temperature falls, the PCM solidifies, releasing the stored energy. Figure 4 shows how PCM can help reduce peak temperatures in electronics cooling solutions.
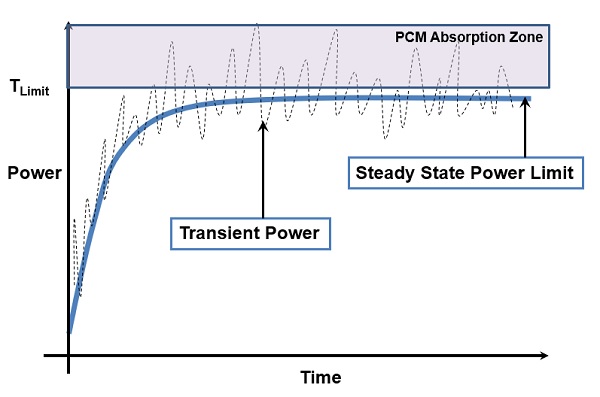
As shown in Fig. 4, PCMs can be used to capture occasional energy spikes coming out of active components. When a PCM with the right melting temperature is used, it melts during periods of high energy use and freezes during periods of low energy use. The end result is that the maximum temperature is maintained around a certain value, near the melting temperature of the PCM.
Since the advent of mobile devices, such as smartphones and tablets, there has been a lot of talk about using PCMs to regulate temperature swings in these devices. However, to date, there is no known such device using PCM. The main problem is space. In smartphones, for example, there is hardly any space left even for very thin heat spreaders. Paraffin, perhaps the most considered PCM for these applications, has low latency. Any meaningful PCM effect on smartphones will require thicknesses of the order of 1 mm. This is too high for a device with an overall device thickness of 7 mm or so, and where thinness is such a premium feature. Other materials such as salt hydrates are just too risky for such applications, because of their undesirable chemical properties (corrosiveness, toxicity, etc.).
PCMs may find wider use in mobile application at some point in the future if there is a breakthrough in material development. For now, their consideration may be limited to some specialty mobile devices where space is not a big factor. Any consideration of PCM for mobile devices or as part of solutions of electronics cooling must involve careful evaluation by the thermal engineer or expert thermal consultants.
From the application side, what makes these devices ideal for PCM use is their ON and OFF operation. In many cases, mobile devices such as smartphones do not operate on a sustained basis. Rather, their use mode generally involves periodic energy spikes followed by almost nothing. In such applications, a PCM can be used to moderate the temperature spikes during periods of high energy usage.
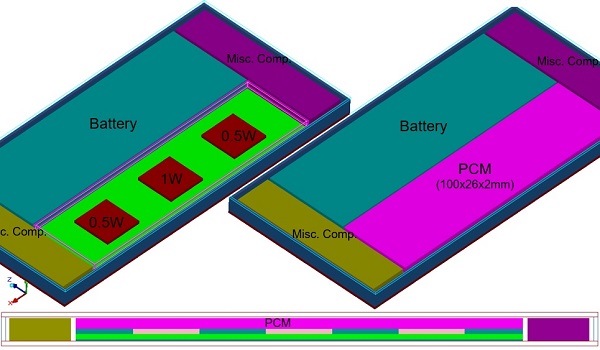
To illustrate this point, we built a simplified smartphone model as shown in Fig. 5. The model includes a block of paraffin PCM, 100x26x2 cubic mm in volume. This PCM was placed right on the top of the main heat-dissipating chips, as shown in Fig. 5. PCMs for such applications come in macroencapsulated forms so that the PCM is self-contained and there is no flow of melt anywhere inside the system. The encapsulant functions as a container as well as a heat spreader. Thin copper foils (as in this case) or thin graphite sheets can be used as encapsulants to compensate for the low thermal conductivity of the paraffin (which is around 0.2 W/mK).
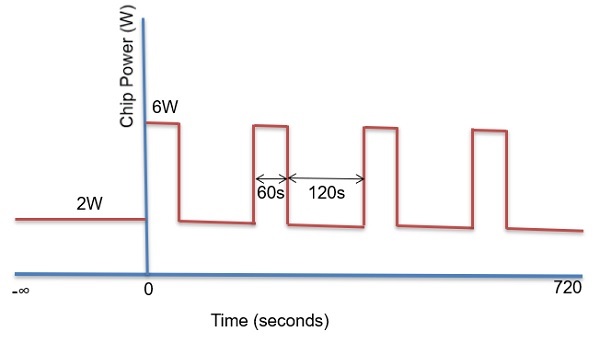
To test the effect of the PCM, we devised a power profile that varied with time, as shown in Fig. 6. The power load profile had 2W at the base and 6W at the peak. Each peak power lasted just 1 minute and the low power 2 minutes. This power was distributed among three chips with one having 50% of the load and the other two having 25% each.
We conducted transient simulations for the entire model with this power profile. In the first run, the PCM body had the thermophysical properties of paraffin, including the latent heat. In the second run, the model had exactly the same layout and properties as the first, but the latent heat capacity was removed from the PCM body. In other words, in the second case, the PCM body does not store any heat.
The results from the two transient thermal simulations are shown below. Fig. 7 shows the temperature variations for the device without PCM. The top curves show the junction temperatures of the powered chips with time. The lower plots in Fig. 7 are the temperature readings at the centers of the top and bottom surfaces of the device – i.e., the display and the back cover. As you can see, the figure shows sharp junction temperature swings because of the power peaks. The skin temperature also shows corresponding variations, mirroring the chip junction temperatures. Further, all the temperature profiles are trending up slightly, indicating a slow build-up of heat inside the system due to insufficient time to cool fully. The peak junction temperature in this case figure ranges from about 84 to 86.5C.
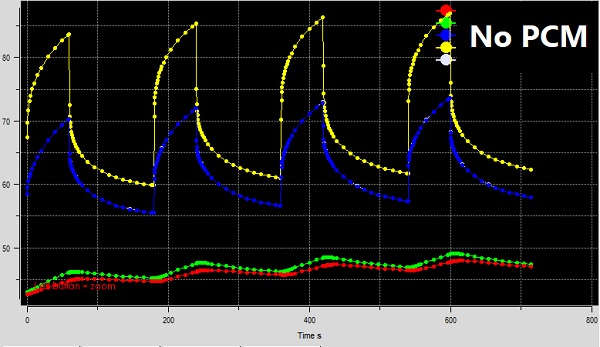
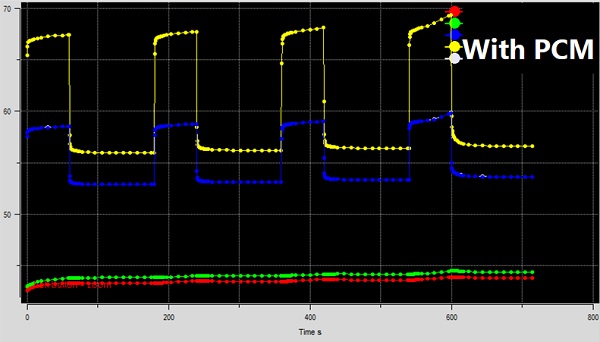
Fig. 8 shows the temperature variations for the model with PCM. The top curves show the junction temperature of the powered chips with time. The lower plots in this figure are the temperature readings at the top and bottom surfaces of the device, i.e., the display and bottom cover. As you can see, in this second run, the junction temperature swings are much more controlled, showing nearly flat temperature profiles except for the last cycle. The skin temperature also shows a nearly flat profile but a slight uptrend overall. The peak junction temperature ranges from about 67.5 to 69C. Compared to the case with no PCM, the peak junction temperature is lower by about 17.5C – a very significant improvement in such devices. Such a significant improvement generally translates into a reduced frequency throttling and a much better user experience. (By frequency throttling, devices are slowed down to reduce overheating.)
Phase Change Materials Sources
There are various companies that supply phase change materials throughout the world. One is Climator from Sweden. This company supplies PCMs for various applications and melting temperatures. One PCM geared for use in thermal management is the Climator C58. This PCM is based on salt hydrates and melts at around 55-58C, good for some applications. It has a latent heat of around 260 KJ/Kg and thermal conductivity of 0.47-0.57 W/mK. It is delivered in Aluminum pouches. It does not bend when solid. They also have PCMs for use in transporting materials, body clothing, and room cooling applications.
Another company supplying a broad range of PCM materials is Rubitherm, based in Germany. This company offers application-oriented PCMs based on your individual specifications. They have all kinds of PCMs geared for buildings, food and beverage, health and medical, logistics, industry, and automotive. The company supplies both encapsulated (micro and macro, including with aluminum case) or unencapsulated PCMs. One of the PCM lineup they have is the RT70HC. This organic (paraffin) material melts in the range 69-71C, has a latent heat of 260 KJ/Kg, thermal conductivity or 0.2 W/mK, and can be used in some electronics cooling solutions.
Croda is another company that supplies PCM for different applications. They manufacture bio-based PCMs that are plant-based, not paraffin or salt hydrate varieties. CrodaTherm PCMs melt to absorb heat and solidify to release heat at defined, specific temperatures. They supply PCMs to diverse applications such as automotive, building and construction, bedding, clothing, food and beverages, and consumer electronics. Most of the PCMs on their list have low melting temperatures, typically in the 20-30C range. But they also have a few like the CrodaTherm™ 60 that melts at around 60C. This is bio-based organic high-temperature PCM derived from plant-based feedstocks that melts around 59.8ºC.
PCM Products Limited is a PCM supplier for a wide range of applications between -100°C (-148°F) and +885°C (+1,625°F) and are available either as the standard PCM solution or in a variety of formats and encapsulated versions. This company probably has more variety among the ones we checked. They have hundreds of PCMs melting at different temperatures, even some as high as 885C. Their product named A118 melts around 118 C and has latent heat of 285 KJ/kg and density of 1450 Kg/m3. Their PlusICE range of products including FlatICE, BallICE, TubeICE, Eutectic Plates, and Pouches enables them to develop and supply both organic and hydrated salt-based PCMs for a variety of applications around the world.

Sample Phase Change Materials
| Company | Product | Melting Temp, C | Latent Heat, KJ/Kg | Thermal Conductivity, W/mK | Density, Kg/m3 | Material Type |
|---|---|---|---|---|---|---|
| Climator | C58 | 55 - 58 | 260 | 0.47 - 0.57 | 1400 | Salt Hydrate |
| Rubitherm | RT70HC | 69 - 71 | 260 | 0.2 | 880 | Organic |
| Rubitherm | RT90HC | 90 - 91 | 170 | 0.2 | 950 | Organic |
| PCM Products Ltd | A28 | 28 | 265 | 0.21 | 789 | Organic |
| PCM Products Ltd | S32 | 32 | 220 | 0.51 | 1460 | Salt Hydrate |
| PCM Products Ltd | A118 | 118 | 285 | 1450 | Organic |
PCM Applications
Today, phase change materials are getting applications in many industries. In addition to electronics cooling, they are being used in buildings, food and beverages, healthcare and medical, logistics, industrial, and automotive applications.

PCM in Buildings
Some buildings are being built with PCMs to help with heating and cooling costs. The PCMs can be embedded inside walls or placed in pellets inside rooms. For example, macroencapsulated PCM boards and cylinders can be installed suspended on ceilings. During warm periods, these boards store energy due to hot air rising. The heat absorbed by the boards is released later during cold periods, warming up the air. Such systems are best suited in countries where the nights are considerably colder than the days, such as in Saudi Arabia. In such places, PCMs on walls and roofs can melt and store large amounts of heat during day time. The heat is then released to the ambient at nights, perhaps with the help of chillers, reducing peak-time electricity costs, which can be very high in some places.
PCM in Food and Beverages
PCMs are being used in the food sector, for transport of food supplies as well as the preservation of prepared foods. For example, using high-melting PCMs, such as RT70HC or GR82 of Rubitherm, one can maintain cooked food from cooling down. Some restaurants and delivery services can take advantage of storage systems made from these materials. Likewise, insulated transport boxes filled with low melting point PCM, such as the RT2HC of Rubitherm, can be used to preserve foods. These packages can keep the food at low temperatures for extended periods of time. This may be necessary in cases where there are technical problems with cooling units and long holding periods before delivery is involved.
PCM in Healthcare and Medical
Phase change materials can used in heat and cold therapy. For example, PCMs can be used to maintain an exact temperature on the skin, irrespective of whether the room is cold or warm. Heat packs are widely used for physical or therapeutic applications. Certain parts of the skin can be cooled without causing hypothermia using a PCM application, for example after accidents or surgeries. PCMs are also used in blankets and sleeping bags to prevent the body from hypothermia, for example, in preparation for surgery or premature baby care where passive means for temporary temperature control are preferred.
PCM in Automotive
PCMs are used in cars for air conditioning and the temperature management of batteries. To reduce fuel consumption and avoid CO2 emissions, some cars are equipped with an automatic start-stop function, where the engine stops at red lights and during waiting times automatically. During this time, the PCM regulates the inside temperature of the car, including the compressor and the engine. The PCM melts when the engine runs and freezes during off times. PCMs are also used in vehicles to manage the temperature of electronic parts, especially battery stacks. This improves the life of lithium-ion batteries whose maximum capacity as well as life is reduced with each charge cycle as well as with large temperature swings. By reducing the temperature variations, battery life and capacity can be improved.
Thermal Analysis with Phase Change Materials
Systems with phase change materials can be easily modeled using thermal analysis packages such as the Ansys Icepak Thermal Analysis software. The analysis is inherently transient and, hence, the full transient thermal simulations must be conducted. This will require inputs such as latent heat, thermal conductivity, and density of the PCM. If these are dependent on temperature, as is often the case, these properties must be entered as functions of the appropriate variable. Care must also be taken in selecting the time steps, as they will greatly affect the quality of the results. For one thing, the time steps must be smaller suring periods where the power load changes rapily. PCM thermal simulations usually require the full conduction and convection solutions within the extended domain inside of which the system resides, such as the one in Fig. 5, shown to demonstrate the effect of PCM on thermal design of mobile devices. If you require further details about this article or you need thermal analysis consulting for your thermal design, please contact us at info@thermalds.com.
Thermal Analysis | Thermal Modeling Consulting
Thermal Design Solutions provides thermal design consulting services in phase change analysis and modeling and thermal management of electronic systems in general. We have decades of experience in many areas of thermal management, working for some well-known technology companies in the world. If you are looking for a thermal management consultants for your project, please contact us at info@thermalds.com. We will happy to discuss your project with you.
Tags: Phase change materials, phase change material, thermal design consultants, thermal consultants, electronics cooling solutions, thermal management consultants, thermal design consulting